
| Uploader: | Vudonos |
| Date Added: | 23 August 2017 |
| File Size: | 11.18 Mb |
| Operating Systems: | Windows NT/2000/XP/2003/2003/7/8/10 MacOS 10/X |
| Downloads: | 95124 |
| Price: | Free* [*Free Regsitration Required] |
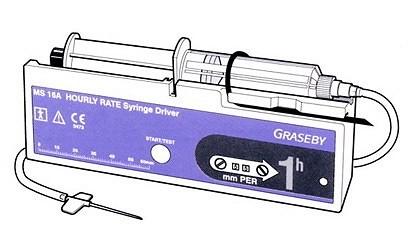
Discontinuation of supply of Graseby MS16A and MS26 syringe drivers
These guidelines reflect international minimum requirements for the safety and effectiveness of grasebyy devices. Graseby MS 16A and MS 26 pumps led to the rapid infusion into the bloodstream of dangerous doses of drugs. Please update your billing details here to continue enjoying your subscription.
Hospices should therefore have acted on the alert at the time. Click here for more information and instructions.
This website graeby Javascript activated in your browser to function. The subscription details associated with this account need to be updated. Start your free trial.
Old-style Graseby syringe drivers (e.g. MS16, MS16A, MS26)
Please update your billing details here to continue enjoying your access to the most informative and considered journalism in the UK. However Medsafe can assist users hraseby providing information about the notification status of alternative devices on the Web Assisted Notification of Devices WAND database and by facilitating end-user group discussions.
Thank you for undertaking this additional check to ensure past safety advice has been fully implemented. Medsafe has commenced consultation with healthcare professionals and stakeholder groups to determine a process and timeline for the removal of all existing Graseby MS-series devices from clinical use.
However, we would urge you to undertake local checks to ensure that none of the old-style Graseby ambulatory syringe drivers, that worked by measuring millimetres of syringe length, are still in use in your organisation e. These syringe drivers are commonly used in palliative care and other situations to provide continuous ambulatory infusion of medicines.
Accessibility Links Skip to content. Medsafe also recognises the on-going risks associated with these devices and therefore advises users to give immediate consideration to sourcing alternative equipment which meets the "Essential Principles" for safety and efficacy.
We would advise that if any of these types of syringe drivers are found to be in use they should be withdrawn as soon as possible; ensuring patient care is not compromised.
This document briefs you on the situation, the reasons for Medsafe's action and the action that will need to be taken by users of these devices. Click here to see more Tap here grraseby see more Tap here to see more. We are aware that large chains may hold medical device asset registers which should provide the necessary information but we know that often these syringe drivers may have been purchased directly by clinical teams using charitable donations and may not have gone through formal organisational purchasing routes.
How to Join NCA? You are currently logged out. We are therefore not envisioning any of these older style syringe drivers to still suringe in use. Please contact NHS Improvement directly with any queries.
Medsafe: New Zealand Medicines and Medical Devices Safety Authority
Alternatively you can ring geaseby office on Without it, key areas of the site will not work for you. We would therefore encourage organisations to directly contact all their clinical teams and units to check if they have any old-style syringe drivers.
Jeremy Hunt, the health secretary, has instructed NHS trusts to remove hazardous syringe drivers from wards. Please update your billing details here. Contact If you have any queries or problems and you would like to speak to one of our team then please fill in the contact form below and we will get back to you as soon as possible.

Want syrjnge read more? Users should consider how best to phase the use of these devices out and consider which device or devices may be used as a satisfactory replacement.
If you have any queries or problems and you would like to speak to one gdaseby our team then please fill in the sgringe form below and we will get back to you as soon as possible. Medsafe recognises the clinical implications of this situation and thus does not currently require existing devices to be recalled or withdrawn from clinical use when alternates are not available provided the manufacturer's instructions are carefully observed. Please update your billing information.
July 1 Already a subscriber or registered access user?

Комментарии
Отправить комментарий